Purified Water System Design
Purified water system design. This section describes design requirements for high purity water systems used to generally supply laboratory sinks and equipment typically know as Reverse OsmosisDe-ionized RODI systems. USP Purified Water Systems. Purified water treatment system design - HOTLINE.
Block and Bleed PUW WFI User Point. Purifying water requires different technologies combining into a single structured system. Nam Trung Viet Environmental Technology Company.
Since endotoxins are produced by the kinds of microorganisms that are prone to inhabit water the equipment and procedures used by the system to purify store and distribute Water for Injection must be designed to minimize or prevent microbial contamination as well as remove incoming endotoxins from the starting water. Water for Injection systems must be. Capacity varying from 100 to 20000 lph litres per hour Compliance with cGMP EurPh USP and JP regulatory requirements or site-specific requirements.
This checks the quality of feedwater which enters the purified. Learn how to maintain high purity water systems from a microbiological aspect. Parental requires very pure water with no endotoxins.
The purity level and volume of water required at each point of use can vary considerably and therefore must be fully assessed in order to properly inform the designer of the water purification system. Total head working for pw distribution pump. Discussion of Pretreatment Part I Pharmaceutical Technology 1994 18 4 38-36.
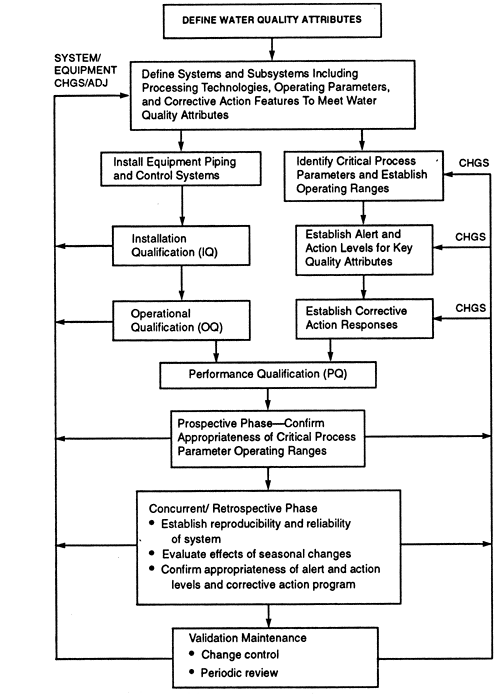
It is widely used as a raw material ingredient and solvent in the processing formulation and manufacture of pharmaceutical products active pharmaceutical ingredients APIs and intermediates and analytical reagents. Our water systems are design to ensure. This is done prior to the design qualification of the system.
Dead legs should be avoided in transporting. To meet purified water microbiological requirements reverse osmosis RO is the preferred technology.
Water for Injection systems must be.
Purified water treatment system design - HOTLINE. Purifying water requires different technologies combining into a single structured system. Capacity varying from 100 to 20000 lph litres per hour Compliance with cGMP EurPh USP and JP regulatory requirements or site-specific requirements. Water should be stored at 75 - 80C. Sanitary diaphragm valves used throughout system Single mechanical seal centrifugal pumps Every line slopes to drain Consider designing system for steaming Recommend envelope gaskets PTFE encapsulated EPDM Construction. E10-03 Our MS Excel Spreadsheet is a MUST HAVE for all facilities having a Purified Water System. However other technologies may also be used. It covers crucial elements including system design system validation microbial limits water for injection systems still heat exchangers holding tanks pumps. Topical and oral products purified water but preservatives in antacids are marginally effective.
Ad Laboratory Ultrapure Water Purification Systems from ELGA LabWater. Design of High-Purity Water Systems 2012 Instructor. 15 internal diameter of pipe. Feed Water is free from pathogens like E- Coli Pseudonymous Salmonella TYPE OF OPERATION. Free shipping for all orders of 150. Thursday 14 November 2013. To meet purified water microbiological requirements reverse osmosis RO is the preferred technology.
%20(5).png?width=600&name=Purified%20Water%20_%20Water%20for%20Injection%20System%20Design%20(Distillation)%20(5).png)


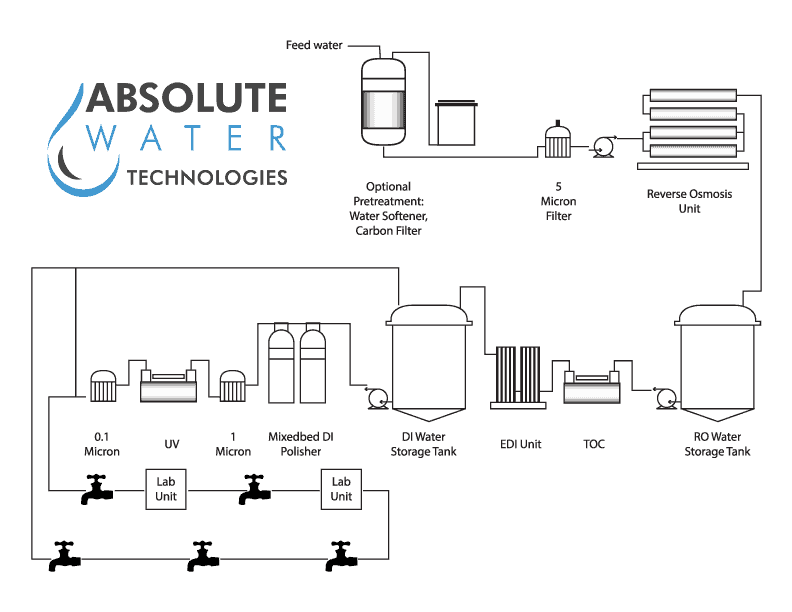




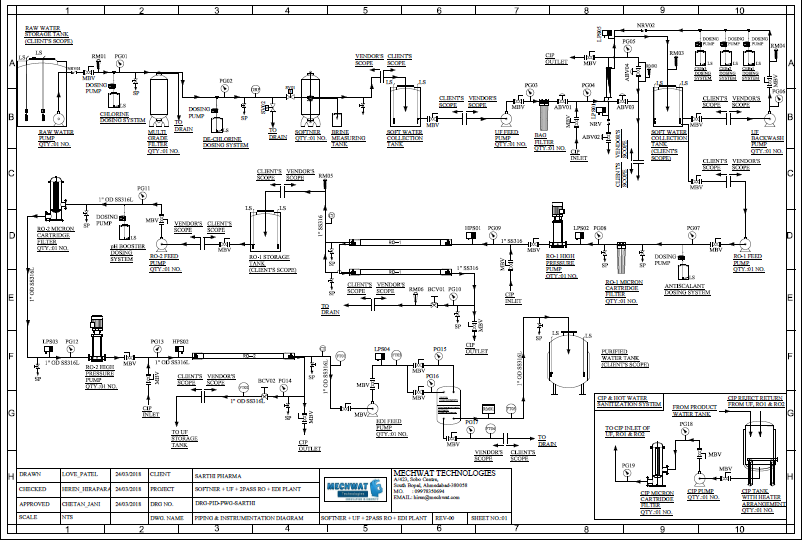

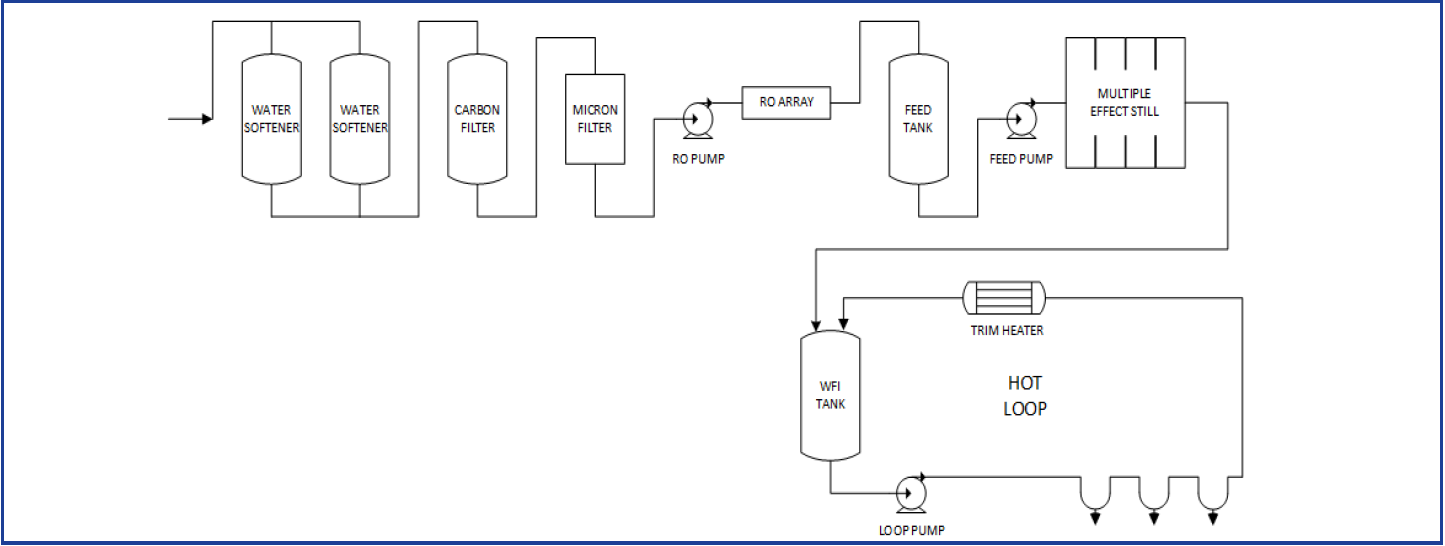

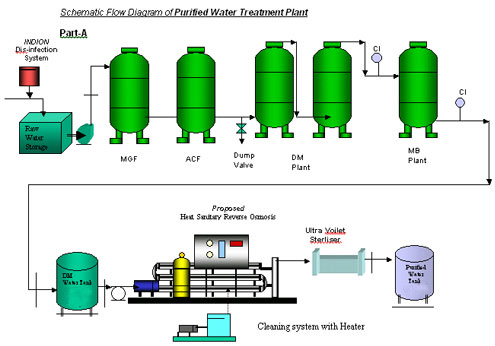











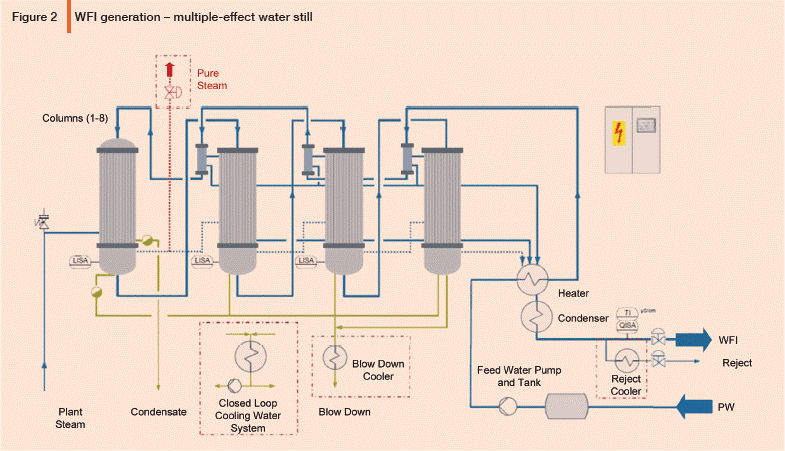









Swjal Process Pvt. Ltd. is a trusted manufacturer of high-purity water treatment solutions, delivering advanced products like Pharmaceutical RO + EDI Systems, DM Water Plants, Ultrafiltration Skids, and WFI Systems. With precision engineering and compliance-driven designs, Swjal ensures reliable purified water generation, storage, and distribution for critical pharmaceutical and biotech applications.
ReplyDeletePure Water Generation System